DEMYSTIFYING HEMP, CBD, CANNABIS AND MARIJUANA AND CONSIDERATIONS ON REGULATIONS
Oct 13, 2018
Industrial hemp-derived oils and other products, commonly referred to as cannabidiol (CBD) products, have grown popular with general consumers and athletes, posing a number of legal, regulatory and consumer questions. Here, we aim to demystify hemp and cannabidiol, or CBD, and evaluate the considerations on whether CBD should be categorized as a drug or a dietary supplement. We invite you to explore the realm of hemp, CBD, Cannabis and Marijuana and considerations on regulations in depth.
DISTINCTIONS BETWEEN CANNABIS AND MARIJUANA
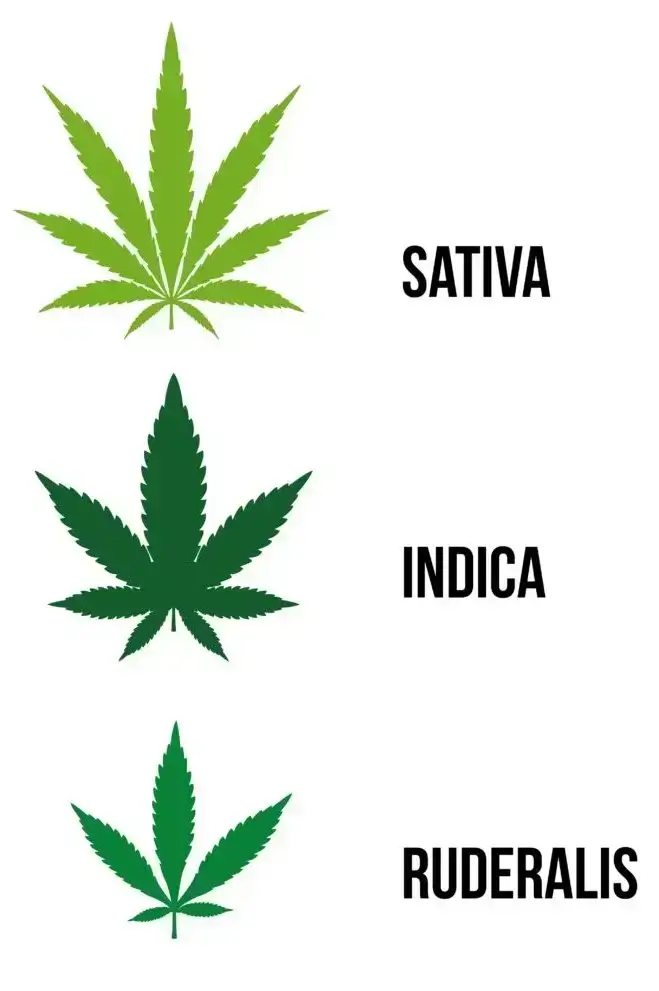 The explanation starts with marijuana. Marijuana has been vilified by many, while also being utilized by others, for hundreds of years. Marijuana is not a scientific term for the plant but rather the general nomenclature typically used for the psychoactive form of cannabis known for getting people high. Cannabis is the more correct general term for the plant, which applies to the cannabis genus in the cannabaceae family, which is part of the plant kingdom. There are three primary species: sativa, indica, and ruderalis. Many hybrid species have developed over the years.
The explanation starts with marijuana. Marijuana has been vilified by many, while also being utilized by others, for hundreds of years. Marijuana is not a scientific term for the plant but rather the general nomenclature typically used for the psychoactive form of cannabis known for getting people high. Cannabis is the more correct general term for the plant, which applies to the cannabis genus in the cannabaceae family, which is part of the plant kingdom. There are three primary species: sativa, indica, and ruderalis. Many hybrid species have developed over the years.
Section 812 of the Controlled Substances Act (21 U.S.C. §801 et seq.) (CSA) lists substances which were controlled in 1970 when the legislation was initiated. Substances may be added, removed, or transferred between schedules annually. The U.S. Department of Justice Drug Enforcement Agency (DEA) Diversion Control Division manages the list of controlled substances. The list includes ‘Marihuana’ and ‘Marihuana extract’ with ‘cannabis’ and ‘marijuana’ also noted. These are all categorized under Schedule I the strictest category of control. Other countries categorize and treat the cannabis plant differently around the world, but we focus here on the state of affairs in the United States.
PHYTOCANNABINOIDS (CANNABINOIDS FOR SHORT) NATURALLY PRESENT IN CANNABIS
To understand the world of cannabis one must have a simple understanding of the chemistry of the plant. Delta 9-Tetrahydrocannabinol (D9-THC) is the most notorious of the phytocannabinoids naturally present in cannabis and is the primary chemical responsible for the psychoactive effects. It is this compound, not the cannabis plant, which should be the focus of regulations.
Hundreds of phytocannabinoids are naturally present in cannabis with the following frequently discussed along with D9-THC: Cannabidiol (CBD), Cannabidiolic Acid (CBDA), Cannabigerol, (CBG), Cannabinol (CBN), Delta 8-Tetrahydrocannabinol (D8-THC), Cannabichromene (CBC), Tetrahydrocannabinolic Acid (THCA). CBD has become the focus of much attention and study for its potential health benefits or possibilities for use as medicine. The other natural phytocannabinoids may also have potential utility.
HEALTH BENEFITS VS. DRUG EFFICACY
Potential health benefits and possible utility as a medicine should not be confused with one another. Dietary supplements and natural products may have potential health benefits while substances that qualify as drugs must have a refined consistent production process and proven medical efficacy for a specific purpose. This distinction needs to be realized when considering the categorization of hemp-derived oil products, also known as CBD products.
HEMP, HEMP SPECIES, AND HISTORICAL USE OF CANNABIS
OK, what is hemp? Hemp in simple terms is a cannabis plant bred and grown to be low in the psychoactive D9-THC. The legal description of industrial hemp in the U.S. mentions cannabis sativa L., but other species of cannabis could also be bred to be below the 0.3 percent D9-THC limit outlined, and high in CBD. Cannabis sativa L is included in the definition of legal hemp as that species historically has a growth pattern more conducive to the production of fiber or seed products. When it comes to the production of hemp with a focus on CBD, other cannabis species may also have relevance.
Hemp itself has been grown for centuries for use in industrial products like rope, paper, seed, food protein, and more. Hemp cultivation has evolved more recently to focus on producing plants that are high in CBD. The historical use of cannabis resins or oils in edible form can be traced back thousands of years to traditional Chinese medicine and other forms of cultural medicine, an important consideration in the proper categorization of hemp-derived products.

CURRENT U.S. HEMP REGULATIONS
So, how has industrial hemp been defined and outlined for use in the U.S.? Two codes apply, one that is current, and the Hemp Farming Act of 2018 introduced in April 2018 by Senator Mitch McConnell from Kentucky that is pending.
U.S. Code – Title 7 – Chapter 88 – Subchapter VII – § 5940 is current. This code establishes the legal confines under which hemp can be legal cultivated. To further demystify the category, we must start with this important statute as written. Important terms are highlighted with italics:
(a) In General – Notwithstanding the Controlled Substances Act (21 U.S.C. 801 et seq.), chapter 81 of title 41, or any other Federal law, an institution of higher education (as defined in section 1001 of title 20) or a State department of agriculture may grow or cultivate industrial hemp if—
(1) the industrial hemp is grown or cultivated for purposes of research conducted under an agricultural pilot program or other agricultural or academic research; and
(2) the growing or cultivating of industrial hemp is allowed under the laws of the State in which such institution of higher education or State department of agriculture is located and such research occurs.
(b) Definitions
In this section:
(1) Agricultural pilot program – The term “agricultural pilot program” means a pilot program to study the growth, cultivation, or marketing of industrial hemp—
(A) in States that permit the growth or cultivation of industrial hemp under the laws of the State; and
(B) in a manner that—
(i) ensures that only institutions of higher education and State departments of agriculture are used to grow or cultivate industrial hemp;
(ii) requires that sites used for growing or cultivating industrial hemp in a State be certified by, and registered with, the State department of agriculture; and
(iii) authorizes State departments of agriculture to promulgate regulations to carry out the pilot program in the States in accordance with the purposes of this section.
(2) Industrial hemp
The term “industrial hemp” means the plant Cannabis sativa L. and any part of such plant, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis.
(3) State department of agriculture
The term “State department of agriculture” means the agency, commission, or department of a State government responsible for agriculture within the State.
INDUSTRIAL HEMP PRODUCTS AND COMMERCE UNDER CURRENT U.S. REGULATIONS
So industrial hemp has been legal to cultivate for research purposes including marketing. Some people might argue marketing equates to sales, but that has been an important element in need of clarification. Under current regulations, cultivation is subject to states permitting the growth of industrial hemp and to the growth occurring at state-registered department of agriculture sites or institutions of higher learning. As of 2018, more than 38 states allow hemp cultivation for commercial, research or pilot program purposes.
The tight set of requirements outlining ‘legal’ hemp cultivation has likely been abused as the industrial hemp product industry has exploded and perhaps co-mingled with the medical and recreational marijuana industries. When it comes to the rules of commerce for the burgeoning industrial hemp oil product industry enormous questions remain.
Nonetheless, a multi-billion dollar industrial hemp oil industry has emerged over the last decade. The terminology used has been a primary challenge. The product sold is industrial hemp oil but the category has been marketed and referred to broadly in commercial practice as the ‘CBD product’ industry. The distinction between industrial hemp oil and ‘CBD products’ must be made in order for clarification of the legality and regulations of this industry.
The industry has expanded based on the notion that ‘CBD products’ may qualify as dietary supplements and could be sold legally across state lines. This is a central component of the debate that we will return to later. The FDA has reiterated, as has the DEA, that sales of ‘CBD products’ or industrial hemp oil products are still considered illegal according to their written regulations. Of course in each state individual laws may also apply. Many states have also questioned the legality of the sale of ‘CBD products’ and industrial hemp oil, including California. Industrial hemp oil has proven hard to categorize due to the terminology challenge.
The enforcement of the regulations has temporarily reached a stalemate and seems to be focused only on those companies that make inappropriate medical claims regarding the benefit of ‘CBD products.’
NEW HEMP FARMING ACT OF 2018 PROPOSES CHANGES IN THE TREATMENT OF HEMP
What does the new Hemp Farming Act of 2018 attempt to do? The important elements are summarized in the discussion below.
First, it clarifies the definition of hemp to specifically include extracts and other cannabinoids as follows: “(1) HEMP.—The term ‘hemp’ means the plant Cannabis sativa L. and any part of that plant, including the seeds thereof and all derivatives, extracts, cannabinoids, isomers, acids, salts, and salts of isomers, whether growing or not, with a delta-9 tetrahydrocannabinol concentration of not more than 0.3 percent on a dry weight basis.” This is important as the current language implies that extracts, oils and cannabinoids would be allowed while the new proposed language specifically includes these items.
Second, it attempts to clarify what sites may be involved in cultivation. It now specifically mentions Native American tribes and has eliminated the specific mention of institutions of higher learning as the current language includes. The Act reiterates that states will be responsible for registering and controlling cultivation sites. It also authorizes the “study of agricultural pilot programs to determine the economic viability of the domestic production and sale of industrial hemp.”
Third, it also allows for important infrastructure like farm insurance to be applied to hemp. Previously cultivation of hemp could not be protected from disaster or other issues like all other industrial crops.
Finally and perhaps most importantly, it proposes to amend the Controlled Substances Act language by clarifying what is controlled. The new proposed language is better but could still use improvement.
CONSIDERATION OF CONTROLLED SUBSTANCES ACT AND INDUSTRIAL HEMP DEFINITION LANGUAGE
The Hemp Farming Act of 2018 proposes to clarify the controlled substances list language in several key ways. To summarize, the term marijuana will now exclude hemp, and ‘Tetrahydrocannabinol’ will be added as Schedule 1, while ‘Tetrahydrocannabinols from hemp’ will be excluded.
The term “tetrahydrocannabinols” was clarified by the DEA in 2003 to refer to both natural and synthetic THC. There are problems with the term as it is non-specific as to the reference of other natural phytocannabinoids.
The proposed changes are a good effort to clarify the language but could be improved as follows. The notation of ‘cannabis’ in the list should be removed as it could still refer to hemp. ‘D9-THC’ should be referenced as the specifically scheduled compound, not ‘tetrahydrocannabinol’, as that term could refer to D8-THC or other phytocannabinoids with ‘tetrahydrocannabinol’ in the name. The listing of ‘tetrahydrocannabinol’ under Schedule I should be replaced with, ‘D9-THC is prohibited in amounts above 0.3% in any product.’ The confusing reference to excluding ‘tetrahydrocannabinols from hemp’ should be replaced with the broader definition ‘phytocannabinoids from hemp.’ ‘Synthetic cannabimimetics’ could be noted to include these important and dangerous compounds. Finally, consideration could be made for expanding the legal definition of industrial hemp to other species of cannabis by simply removing the words ‘sativa L.’ from the definition of industrial hemp.
GW PHARMACEUTICALS CANNABIS-DERIVED DRUGS – EPIDIOLEX AND SATIVEX
The controlled substance list language is important, particularly given the recent FDA approval of GW Pharmaceuticals cannabis-derived drug Epidiolex. The approval of Epidiolex has created even more questions in the debate over whether CBD should be considered a drug or a supplement. So what is Epidiolex, which has been approved for the treatment of seizures, and its cousin Sativex, which is in clinical trials for spasticity?
Epidiolex (cannabidiol, CBD) is described at Epidiolex.com as follows: “EPIDIOLEX contains highly purified, plant-derived cannabidiol; the active ingredient is nearly 100% cannabidiol. EPIDIOLEX is manufactured with the highest standards of consistency, so the formulation is the same with every prescription.” In summary, Epidiolex is almost pure CBD with trace amounts of other phytocannabinoids. Sativex® (delta-9-tetrahydrocannibinol and cannabidiol in the EU) (nabiximols in the USA), meanwhile, is described as “delta-9-tetrahydrocannibinol (THC) and cannabidiol (CBD) in a 1:1 ratio as well as specific minor cannabinoids and other non-cannabinoid components.” The naming convention of Epidiolex which refers to cannabidiol and CBD in the parentheses are very important to the drug vs. supplement discussion that is ongoing, which is largely focused on the CBD nomenclature.
In order to make room in the regulations for the GW Pharmaceuticals cannabis-derived drugs the controlled substances list language had to be adjusted to include them. This was accomplished as follows. The list moved “Approved Cannabidiol Drugs” to Schedule V with the following vitally important note under Other Names, “A drug product in finished dosage formulation that has been approved by the U.S. Food and Drug Administration that contains Cannabidiol derived from cannabis and no more than 0.1 percent (w/w) residual tetrahydrocannabinols.”
FULL SPECTRUM HEMP OIL AND THE DIFFERENCE BETWEEN INDUSTRIAL HEMP OIL AND CANNABIS-DERIVED DRUGS
Full spectrum hemp oil is another key term. Full spectrum hemp oil should refer to an oil that contains a natural spectrum of phytocannabinoids that are present in the cannabis plant used for extraction. It would be expected to contain a mixture of D9-THC, CBD, and other phytocannabinoids in whatever ratio is present in that particular cannabis plant.
Neither of the GW Pharmaceuticals drugs, Epidiolex or Sativex, is cannabis resin or oil in its natural full spectrum form. Both have been highly processed for a specific and consistent amount of CBD, D9-THC in the case of Sativex, or other components as outlined and are prescribed for use according to defined dosages. This is important when one returns to the distinction as to whether CBD should be considered a drug or a dietary supplement.
AN ARGUMENT FOR HOW INDUSTRIAL HEMP OIL QUALIFIES AS A LEGAL DIETARY SUPPLEMENT INGREDIENT
The argument rages as to whether industrial hemp derived oil should qualify as a legal dietary supplement ingredient. In order to understand the argument one must understand the definition of a dietary supplement ingredient in the U.S. as defined in the Dietary Supplement Health and Education Act (DSHEA) of 1994; important excerpts are below.
(ff) The term “dietary supplement –
(1) means a product (other than tobacco) intended to supplement the diet that bears or contains one or more of the following dietary ingredients:
(A) a vitamin;
(B) a mineral;
(C) an herb or other botanical;
(D) an amino acid;
(E) a dietary substance for use by man to supplement the diet by increasing the total dietary intake; or
(F) a concentrate, metabolite, constituent, extract, or combination of any ingredient described in clause (A), (B), (C), (D), or (E);…
NEW DIETARY INGREDIENTS SEC. 413. (a) IN GENERAL. – A dietary supplement which contains a new dietary ingredient shall be deemed adulterated under section 402(f) unless it meets one of the following requirements:
(1) The dietary supplement contains only dietary ingredients which have been present in the food supply as an article used for food in a form in which the food has not been chemically altered.
(2) There is a history of use or other evidence of safety establishing that the dietary ingredient when used under the conditions recommended or suggested in the labeling of the dietary supplement will reasonably be expected to be safe and, at least 75 days before being introduced or delivered for introduction into interstate commerce, the manufacturer or distributor of the dietary ingredient or dietary supplement provides the Secretary with information, including any citation to published articles, which is the basis on which the manufacturer or distributor has concluded that a dietary supplement containing such dietary ingredient will reasonably be expected to be safe.
Industrial hemp derived oil certainly seems to qualify as a dietary supplement based on this language. Industrial hemp oil is an extract of the cannabis plant. Some researchers suggest the oil could be used to supplement the diet and influence the endocannabinoid system present in the human body. Cannabis resin or oil has been in the food supply prior to 1994 in a form not chemically altered as it has been used historically for centuries as an edible in a variety of cultural practices. There is a great deal of literature on the historical use of cannabis with one notation reading, “Smoking did not become common in the Old World until after the introduction of tobacco, so up until the 1500s hashish in the Muslim world was consumed as an edible.” The chemicals in the cannabis plant remain the same today.
RESOLVING THE QUESTION AS TO WHETHER CBD IS A DRUG OR SUPPLEMENT
The approval of the GW Pharmaceuticals cannabis-derived drugs has complicated the situation as the terms ‘cannabidiol’ and ‘CBD’ are now associated with the naming convention of the drug Epidiolex. Meanwhile, the dietary supplement industry has predominantly elected to market industrial hemp-derived oil products as ‘CBD products.’ This is why confusion has grown over categorization of CBD as it is now marketed as both a drug and a supplement.
The actual material is very different. The drugs are highly standardized phytocannabinoids produced to specific purity and/or ratio specifications. The DEA controlled substances list language dated October 1, 2018 defines “Approved Cannabidiol Drugs” as “a drug product in finished dosage formulation that has been approved by the U.S. Food and Drug Administration that contains Cannabidiol derived from cannabis and no more than 0.1 percent (w/w) residual tetrahydrocannabinols.” Industrial hemp oil is prepared in unspecified, non-specific ratios and is expected to be lower in CBD purity and content with higher amounts of other phytocannabinoids than Epidiolex. So, industrial hemp oil does not seem to comport with the definition of a cannabidiol drug used by the DEA.
Even though both are made with phytocannabinoids a distinction should be made between the specific ratios of phytocannabinoids used in the cannabis-derived drugs and the non-specific industrial hemp oil that has scientific merit to be legally categorized as a dietary supplement ingredient under the current DSHEA language. The ideal terminology to consider for dietary supplement ingredients derived from cannabis should include: ‘industrial hemp oil,’ ‘phytocannabinoids from industrial hemp,’ and ‘full spectrum industrial hemp oil.’
Cannabinoid profile testing can be used to distinguish between industrial hemp oil dietary supplement ingredients and the specific drugs Epidiolex and Sativex. So there is a scientific solution but what about a terminology solution? Could the term ‘CBD’ be isolated to refer to Epidiolex while the dietary supplement industry uses the language suggested above to differentiate the two categories? Yes, but ‘CBD’ has become so engrained already within the terminology of the dietary supplement industry changing its use now would prove challenging.
Whether the drug or dietary supplement industry gets to lay claim to the term ‘CBD’ and how that relates to marketing seems to be the crux of the remaining argument, as well as the controlled substances list language. The existing regulations as written should be able to distinguish the two categories as outlined herein although this remains to be clarified. Meanwhile, the industrial hemp oil industry continues to expand and grow despite the regulatory quandary on legality and categorization.
QUALITY CONTROL CONCERNS AND INTRODUCTION OF THE BSCG CERTIFIED HEMP PROGRAM
<
 With the growth of the category has come expanded quality control concerns. Do industrial hemp oil products adhere to good manufacturing practices (GMP)? Are they properly tested for contaminants like pesticides, heavy metals or solvents? Do the products really test below the legal 0.3 percent D9-THC limit outlined? Are any dangerous synthetic cannabimimetics being substituted for natural phytocannabinoids? Could these products make an athlete fail a drug test? All of these are good questions.
With the growth of the category has come expanded quality control concerns. Do industrial hemp oil products adhere to good manufacturing practices (GMP)? Are they properly tested for contaminants like pesticides, heavy metals or solvents? Do the products really test below the legal 0.3 percent D9-THC limit outlined? Are any dangerous synthetic cannabimimetics being substituted for natural phytocannabinoids? Could these products make an athlete fail a drug test? All of these are good questions.
At BSCG, we don’t just aim to outline problems; we like to offer solutions! In that effort, BSCG is pleased to introduce our new third-party certification program BSCG Certified Hemp™. We are excited to provide a new level of transparency and quality control to the industrial hemp product industry and help address important consumer concerns in the process.
– Oliver Catlin, BSCG President